Abstract
Background Isocitrate Dehydrogenase (IDH) 1 or 2 mutations occur in ~20% of acute myeloid leukemia (AML). Both venetoclax (VEN)-based and targeted IDH-inhibitor (IDHi) therapies are effective treatment options for IDH mutated AML in combination with hypomethylating agents (HMA). ASTX727 is an oral, fixed dose (35 mg/100 mg) combination of decitabine and cedazuridine approved for the treatment of myelodysplastic syndrome (MDS). Herein we report the interim results of the first all-oral triplet regimen of ASTX727 (day 1-5) + VEN (day 1-14) in combination with a targeted mutant IDH1i ivosidenib (IVO) or IDH2i enasidenib (ENA), for IDH mutated AML.
Methods Eligible patients were > 18 years old with relapsed/refractory (R/R) IDH1 or IDH2 mutated AML, or newly diagnosed (ND) AML not eligible for intensive chemotherapy. For the R/R AML cohort, prior VEN, HMA or IDHi use was not exclusionary. The primary objectives were to determine safety and the recommended phase 2 dose (RP2D) of ASTX727 and VEN with ivosidenib (Arm A) or enasidenib (Arm B) [Phase 1b], and to determine the composite remission rate (CRc; CRh+CRi+CR) for both arms [Phase 2].
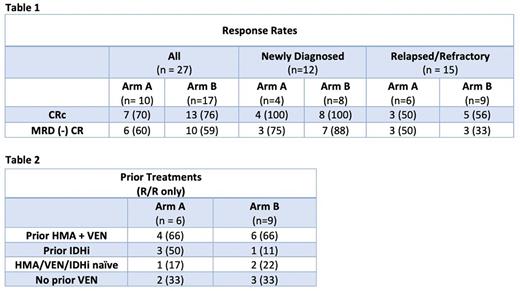
Results A total of 32 patients have enrolled, with 3 screen failures and 2 patients ongoing first cycle of therapy. There are currently 27 evaluable patients (arm A: 10, arm B: 17) with median follow up of 5.7 months. Median age at enrollment was 73 (50 - 81). 44% (n=12) had ND AML and 56% (n=15) had R/R AML. European LeukemiaNet (ELN) risk was intermediate or adverse in 52% and 44%, respectively. Patients received a median of 4 treatment cycles (ND: 5, R/R: 3). The CRc rate in the overall population was 72% (ND: 100%, R/R: 53%). Measurable residual disease (MRD) negative CRc by flow cytometry was achieved in 64% (ND: 83%, R/R: 42%). (Table 1) In the R/R cohort, 78% (n=11) patients had received prior treatment with either an HMA (azacitidine or decitabine), BCL2i and/or IDHi, with a median of 2 prior treatments. (Table 2) 5 patients did not have prior VEN exposure of which 4 (80%) achieved an MRD negative CRc. Four patients transitioned to stem cell transplant (ND: 2, R/R: 2).
The most common non-hematologic grade 3/4 AEs in the overall population included hyperbilirubinemia (n=2, 7%), representing indirect bilirubin in enasidenib-treated patients and mucositis (n=2, 7%) with one considered a residual effect of prior cytoreduction. There were two patients with possible/probable differentiation syndrome which resolved with medical management (dexamethasone and diuresis). Two episodes of reversible grade 1 tumor lysis syndrome were also reported. 60-day mortality within the ND and R/R cohort was 8% (n=1) and 0%, respectively. The ND patient withdrew from the study and went home on hospice after an admission for febrile neutropenia.
Conclusions The combination of ASTX727 + VEN with IDH1 or IDH2 inhibition appears to be an effective regimen for the treatment of IDH mutated AML with high response rates including MRD-negative CRc, most notably in de novo patients and R/R pts without prior VEN exposure. AEs are anticipated and tolerable. Enrollment to this study is ongoing.
Disclosures
Sasaki:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Alvarado:BerGenBio: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Takahashi:Ostuka Pharmaceuticals: Honoraria; Agios: Consultancy; GSK: Consultancy; Celgene/BMS: Consultancy; Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy; Mission Bio: Honoraria; Illumina: Honoraria. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Ferrajoli:Beigene: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Short:Takeda Oncology: Consultancy, Research Funding; Stemline Therapeutics: Research Funding; Astellas: Research Funding; AstraZeneca: Consultancy; Amgen: Consultancy, Honoraria; Novartis: Consultancy; Pfizer: Consultancy. Ravandi:Biomea Fusion, Inc.: Research Funding; AstraZeneca: Consultancy; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Amgen: Honoraria, Research Funding; Prelude: Research Funding. Jabbour:Bristol Myers Squibb: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding. Andreeff:Chimerix: Current holder of stock options in a privately-held company; Oncolyze: Current holder of stock options in a privately-held company; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Brooklyn ITX: Research Funding; Kintor Pharmaceutical: Research Funding; Breast Cancer Research Foundation: Research Funding; Reata: Current holder of stock options in a privately-held company; Syndax: Consultancy, Research Funding; Senti Bio: Consultancy, Research Funding; Pinot Bio: Research Funding; Oxford Biomedical UK: Research Funding; AstraZeneca: Research Funding; Glycomimetics: Consultancy; Medicxi: Consultancy; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; NCI: Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Acceleron Pharma: Consultancy; Genentech: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Aprea: Honoraria; Curis: Honoraria, Research Funding; Gilead Sciences: Research Funding. Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; NOVA Research: Honoraria; Jazz Pharmaceuticals: Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria. Konopleva:Cellectis: Research Funding; Eli Lilly: Consultancy, Honoraria, Research Funding; Stemline therapuetics: Consultancy, Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Forty Seven: Honoraria, Research Funding; Sanofi: Research Funding; Rafael Pharmaceutical: Research Funding; AstraZeneca: Research Funding; Ascentage: Research Funding; Ablynx: Research Funding; Calithera: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. DiNardo:Novartis: Honoraria; Forma: Research Funding; Astellas: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cleave: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; AbbVie: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Astex: Research Funding; Foghorn: Honoraria, Research Funding; Gilead: Honoraria; LOXO: Research Funding; Bluebird Bio: Honoraria; Servier: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal